

Tell FDA Vinpocetine Must Stay!
Tell FDA Vinpocetine Must Stay!
Take Action to keep public access to this dietary supplement ingredient! Comment by November 7.
The FDA wants to remove a widely used dietary ingredient, vinpocetine, from the public domain! Though this is not an unprecedented move, the rationale is new: relying on the NDI Draft Guidance to exclude a substance with numerous successful NDI notifications on file from the definition of a dietary supplement.
Thanks to our colleagues at Life Extension Foundation, you can easily Take Action here to send the FDA a public comment protesting their attempt to rob you of vinpocetine and simultaneously send your Representative and Senators a letter asking them to stop the FDA from implementing this and other proposed guidelines that will cause many popular dietary supplements to be banned. Take Action here. See notice in Federal Register.
The safety and efficacy of vinpocetine is not in question by the agency. The notice goes against Congress’s specific intent in the 1994 Dietary Supplement Act (DSHEA) that “the [FDA] should not take any actions to impose regulatory barriers limiting the flow of safe products to consumers.” There are 600 published references attesting to the safety and benefits of vinpocetine on the National Library of Medicine’s database (pubmed.gov). Take Action here.
RECENT NEWS

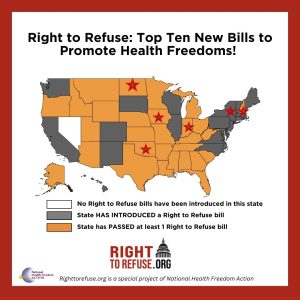
Right to Refuse: Top Ten New Bills to Promote Health Freedoms!


